Compliance is key
Businesses in the life sciences industry typically manage a large volume of sensitive data, such as patient records, drug specifications, and clinical research. Mishandling of this data can result in:
- Penalties
- Prosecution for negligence
- Warning letters
- Denial of new drug applications
- Delay in manufacturing
- Reputational damage
- Recalls
- Seizure of drugs and devices
The FDA's CFR in the United States and the EU GMP in the European Union establish strict regulations for the handling of sensitive data to ensure confidentiality.
Popular fields
- Drugs and pharmaceuticals
- Food and beverage
- Medical devices
- Tobacco products
- Cosmetics
- Radiation-emitting devices
- Animal and veterinary
- Vaccines, blood, and biologics
- Dietary supplements
- Food and color additives
Popular use cases
- Patient consent forms
- Hiring and onboarding documents
- Research documents
- Procurement forms
- Non-disclosure agreements
- Certificates
- Reports
- Compliance documents
Enable Zoho Sign's controls for life sciences in a few clicks!
- STEP 1
Click Settings in the left navigation pane.
- STEP 2
Go to Account Settings.
- STEP 3
Toggle Controls for life sciences to ON.
- STEP 4
Agree to the disclosure and click Enable
Key benefits
- Easy compliance and validation
- Tighter authentication to access and sign documents
- Minimized risks and lowered costs
- Seamless signing experience
- Automated workflows
What is 21 CFR Part 11?
This is a set of federal regulations to be followed by fields governed by the FDA, such as those in the life sciences industry. Title 21 Part 11 defines electronic records and electronic signatures, and further lays down the criteria by which they may be deemed trustworthy, reliable, and equivalent to wet ink signatures.
What is EU Annex 11?
This guideline establishes the practices that can be adopted in the manufacturing of medical products for human and veterinary use. In particular, Annex 11 provides guidelines for the implementation, use, and maintenance of computerized systems used in the human and veterinary medical industry.
Stay compliant with Zoho Sign
Zoho Sign provides controls and features that ensure your digital signatures and signed documents are compliant with global regulations.
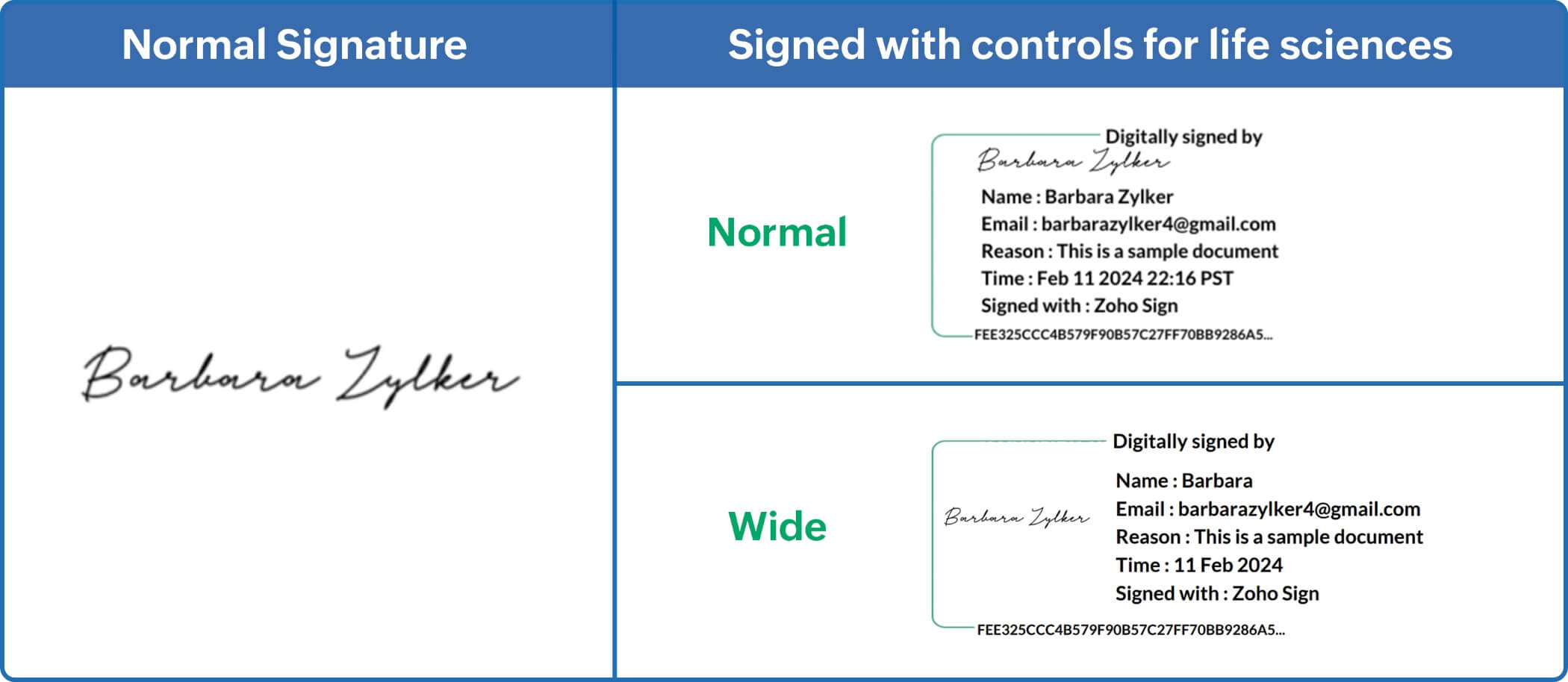
- Visible signatures with metadata
- ID verification of signers
- Security, control, and validation
- Customized legal disclosures
- Free onboarding and training sessions
Frequently asked questions:
In which Zoho Sign edition is the life sciences module available?
You can enable controls for life sciences in the Enterprise plan on request. Please email support@zohosign.com to request this feature.
What controls are applied when I enable controls for life sciences?
Enabling controls for life sciences in Zoho Sign will automatically activate some features while restricting a few others. Read about these controls in detail here.
How do I validate the life sciences module?
Zoho Sign's life sciences module comes with validation documents that you can access upon request by signing a non-disclosure agreement.



